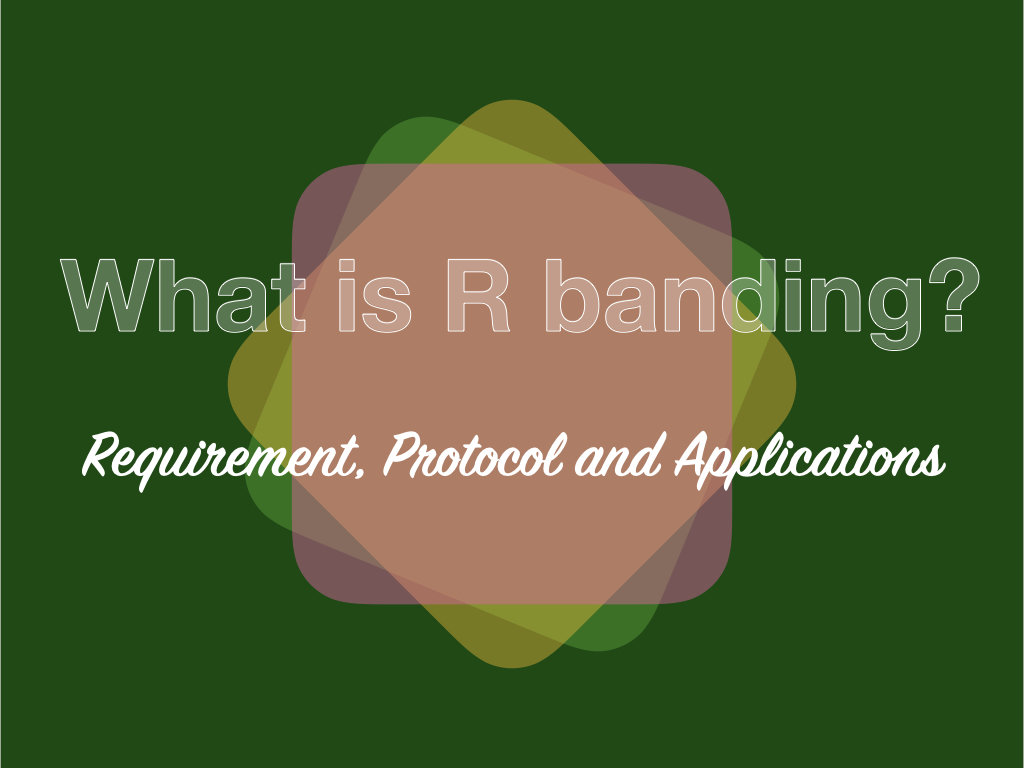
The R banding is reverse banding or reverse Giemsa banding used to stain the regions of GC, a complement to G bands.
We are exploring varied chromosome banding techniques that are used for various clinical investigations and other applications. So far we have discussed, GTG banding, FQF banding, C banding, NOR banding and T banding.
To observe and investigate different anomalies a different banding technique is used. We also have given protocols for every banding in each article.
This article focus on the reverse banding or R banding technique, which literally shows It might be a reverse process of something!
We will explain the mechanism of Reverse banding, process, protocol and applications in this article.
Read more: Interpreting human karyotype report.
What is R banding?
In 1971, Lejeune and Dutillaux had developed the native R banding technique, however, timely changes were made in it.
Reverse banding by Giemsa, reverse banding by quinacrine and reverse banding by chromomycin A3 or olivomycin are applied often.
Principle:
The present banding technique is based on the heat denaturation of DNA and proteins.
The regions that are stained light pink using the GTG banding are stained darker using the reverse banding. Differential R banding using the fluorescence dye can be stained.
Heat denatures the loosely packed AT-rich region but not the GC-rich which stains darker. A differential pattern of chromosome banding reverse to either G or Q banding develops.
Requirements:
Water bath, coupling jars, Giemsa solution or stain, Phosphate buffer, Slides and coverslip, acridine orange dye and fluorescence microscope.
Protocole:
R banding using Giemsa:
- Preheat phosphate buffer saline in coupling jar at 85°C temperature for few minutes.
- Incubate slides for 5 to 10 minutes in it.
- Rinse slides with water and stain with Giemsa solution (used in the GTG banding) for 10 minutes.
- Wash with distilled water and observe under the microscope.
- Note that pH of phosphate buffer has a crucial role in final results. To know more about the pH and how to prepare it, download our ebook when available. (pH is 6.5).
R banding using acridine orange dye:
Preheat coupling jar having 50ml phosphate buffer at 85°C temperature for 10 minutes.
Wash the slides and stain with the acridine orange dye at room temperature for few minutes and wash with buffer.
Cover it with a clean coverslip and observe immediately under the fluorescence microscope.
Caution: acridine orange is a fluorescent dye and a possible mutagen, handle it with care.
Note that incubate for a shorter time if slides are aged. Slides aged at room temperature for 1 to 2 weeks give excellent results with the present protocol.
Applications:
Reverse banding isn’t a specialized one! Means, can’t be used for a specific purpose, it is just a reverse process of existing banding pattern.
Note that, some laboratories and researchers prefer to use R banding instead of G or Q banding in their routine examination.
However, the critical application of the present technique is in examining some telomeric bands which are visible faintly using either G bands or Q bands, are stained darkly.
Besides, scientists prefer reverse banding to evaluate and cross verify the interpretations or results of clinical indications.
Other banding techniques: Q-Banding, C-Banding, T-Banding.
Conclusion:
The clear mechanism of r banding isn’t known yet. The reason why heating shifts the Giemsa banding pattern in the case of R banding is a subject to research.
R banding isn’t significant for day to day clinical investigation still, as a cytogeneticist we should learn it.
Comment below and let us know your thoughts on the present topic.